
You are here: Home >> History of Light >> Quantum Mechanics >> ...from A. Einstein
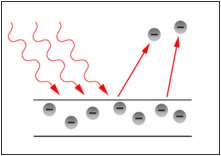 The Photoelectric Effect was discovered in 1887 by a German physicist, Heinrich Rudolf Hertz, who observed that ultraviolet light changes the lowest voltage at which sparking takes place between given metallic electrodes. At the close of the 19th century, it was established that a cathode ray (produced by an electric discharge in a rarefied-gas atmosphere) consists of discrete particles, called electrons, each bearing an elementary negative charge.
The Photoelectric Effect was discovered in 1887 by a German physicist, Heinrich Rudolf Hertz, who observed that ultraviolet light changes the lowest voltage at which sparking takes place between given metallic electrodes. At the close of the 19th century, it was established that a cathode ray (produced by an electric discharge in a rarefied-gas atmosphere) consists of discrete particles, called electrons, each bearing an elementary negative charge.
In 1900 Philip Lenard, a German physicist, studying the electrical charges liberated from a metal surface when it was illuminated, concluded that these charges were identical to the electrons observed in cathode rays. It was further discovered that the current (given the name photoelectric because it was caused by light rays), made up of electrons released from the metal, is proportional to the intensity of the light causing it for any fixed wavelength of light that is used. In 1902 it was proved that the maximum kinetic energy of an electron in the photoelectric effect is independent of the intensity of the light ray and depends on its frequency.
 The observations that (1) the number of electrons released in the photoelectric effect is proportional to the intensity of the light and that (2) the frequency, or wavelength, of light determines the maximum kinetic energy of the electrons indicated a kind of interaction between light and matter that could not be explained in terms of classical physics.
The observations that (1) the number of electrons released in the photoelectric effect is proportional to the intensity of the light and that (2) the frequency, or wavelength, of light determines the maximum kinetic energy of the electrons indicated a kind of interaction between light and matter that could not be explained in terms of classical physics.
The search for an explanation led in 1905 to Albert Einstein's fundamental theory that light, long thought to be wavelike, can be regarded alternatively as composed of discrete particles (photons), equivalent to energy quanta. This work brought to A. Einstein the Nobel Prize.
Quantum theory was soon present almost everywhere, but it did not seem to be interesting for Einstein himself. When he felt he had been convinced for the existence of his ideas he abandoned Quantumechanics. On the contrary a Danish scientist, N. Bohr was about to announce some new concepts about the formation of atoms.
Related links (external pages)